Formic acid 64-18-6
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
Formic acid 64-18-6

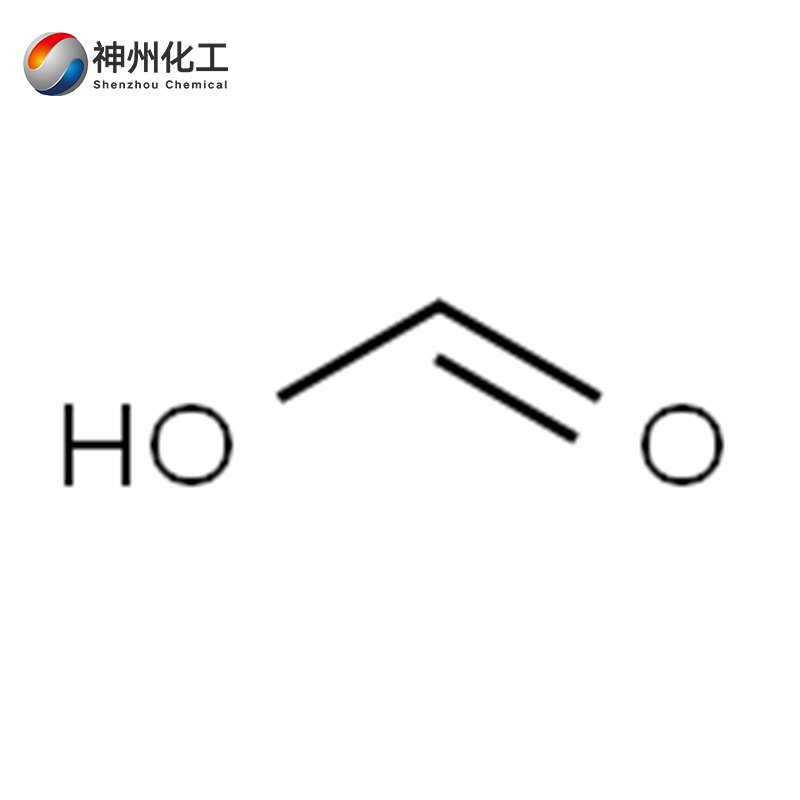


- Chemical Name:Formic acid 64-18-6
- CAS No.:64-18-6
- Product Categories:Food Additives
- Molecular Formula:CH2O2
- Formula Weight:46.03
- Appearance:Colorless liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:Formic acid 64-18-6
- CAS No.:64-18-6
- Product Categories:Food Additives
- Molecular Formula:CH2O2
- Formula Weight:46.03
- Appearance:Colorless liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available




Product Description of Formic acid 64-18-6
Formic acid 64-18-6 is an important chemical raw material, first discovered by Fisher in 1670. In 1749, A.S. Marggret first produced pure formic acid. It was named formic acid because it was first discovered through the distillation of red ants. Formic acid is widely present in nature, such as in the secretions of red ants, bees, and caterpillars, in the leaves and roots of plants, and in fruits. It is the simplest carboxylic acid, with a unique structure and stronger acidity compared to other fatty carboxylic acids.
It is a colorless, transparent, fuming liquid with a pungent odor, with the molecular formula HCOOH. Its relative molecular mass is 46.03. Its relative density is 1.2196. Its melting point is 8.4°C. Its freezing point is 7°C. Its boiling point is 100.7°C at 50°C (15.999 × 10³ Pa). Refractive index: 1.3714. Flash point: 68.9°C. Viscosity: 1.784 mPa·s. Ignition point: 410°C. Miscible with water, ethanol, ether, etc.
Forms a high-boiling-point binary azeotrope with water, containing 77.5% of this compound, with an azeotropic boiling point of 107.3°C, which is higher than the boiling points of pure water and pure formic acid. The carbonyl group of this compound is directly bonded to hydrogen, giving formic acid both acidic properties and some characteristics of aldehydes. It can form salts and esters; react with amines to form amides; and undergo addition reactions with unsaturated hydrocarbons to form esters.
Its reducing properties reflect those of an aldehyde, such as reducing silver nitrate in ammonia solution (Toulon reagent) to metallic silver, decolorizing potassium permanganate, reducing sulfur dioxide to thiosulfate ions (SO₂ → S₂O₃²⁻), and reducing mercury nitrate to mercury. Formic acid reacts with concentrated sulfuric acid to dehydrate and produce carbon monoxide. Heating to 160°C can also cause decarboxylation, producing carbon dioxide and hydrogen; under the catalysis of copper-chromium, platinum, palladium, etc., decomposition reactions can also occur, producing carbon dioxide and hydrogen.
This product is highly irritating and corrosive. Inhaling its vapors can irritate the respiratory system and cause inflammation; contact with the skin can cause blistering. Chronic poisoning can lead to hematuria. The oral LD50 in rats is 1210 mg/kg. The maximum allowable concentration in the workplace is 5 × 10⁻⁶. When storing, keep away from fire sources and heat sources. It should be isolated from oxidizers, alkalis, strong acids, H-perforating agents, and cyanides. Handle with care during loading and unloading; do not invert. Prevent condensation and damage to packaging.
Spilled material should be rinsed with water, but protective gear must be worn during handling. In case of skin contact, immediately rinse with copious amounts of water and apply ointment if necessary. In case of inhalation poisoning, seek medical attention immediately.
Chemical Properties of Formic acid 64-18-6
| Melting point | 8.2-8.4 °C (lit.) |
| Boiling point | 100-101 °C (lit.) |
| density | 1.22g/mL (lit.) |
| vapor pressure | <1 Pa (25 °C) |
| refractive index | n20/D 1.430(lit.) |
| Fp | 133 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | water: miscible |
| form | Liquid |
| pka | -0.44±0.70(Predicted) |
| color | APHA: ≤15 |
| Odor | Faint, ammonia-like odor detectable at 100 ppm |
| Water Solubility | soluble |
| Merck | 144241 |
| BRN | 1209246 |
| Dielectric constant | 36.710000000000001 |
| InChIKey | ZMXDDKWLCZADIW-UHFFFAOYSA-N |
| LogP | -0.540 |
| CAS DataBase Reference | 64-18-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Formic acidl-(64-18-6) |
| EPA Substance Registry System | Formic acid (64-18-6) |
Application of Formic acid 64-18-6
Formic acid 64-18-6 is one of the basic organic chemical raw materials, widely used in the pesticide, leather, textile, dyeing, pharmaceutical, and rubber industries, among others. It can also be used to produce various solvents, plasticizers, rubber coagulants, animal feed additives, and insulin synthesized using new processes. In China, the pharmaceutical industry accounts for approximately 45% of formic acid consumption, the chemical industry accounts for approximately 30%, and the light industry, textile industry, and other sectors account for approximately 25%. Formic acid is one of China’s important export chemical products.
Currently, all formic acid production in China uses the sodium formate method. Formic acid is commonly used as a cheap substitute for inorganic acids with lower volatility and corrosion, and is widely used in the light industry. In the textile and dyeing industries, formic acid is used as an agent to eliminate nitrous acid gas produced by the sodium nitrite method, as a dyeing aid for weak acidic dyes and neutral complex dyes, and as a dyeing aid for reactive dyes on nylon. Formic acid does not remain on the fabric during the dyeing process. It has a stronger acidity than acetic acid and can reduce hexavalent chromium, thereby improving dye utilization in the chromium mordanting process.
Replacing sulfuric acid with formic acid avoids cellulose degradation, and its moderate acidity ensures uniform dyeing, making it an excellent dyeing aid. Formic acid is used as a substitute for inorganic acids in tanning for decolorization, hair removal, neutralizing lime, and preventing mold growth in wet leather. Using formic acid as a coagulant improves the quality of natural rubber, reduces production costs, and can also be used for the regeneration of waste rubber. Formic acid has great potential as a feed additive in silage feed.
Formic acid has the function of inhibiting or preventing mold growth, altering the natural fermentation form of feed, and is often added with fatty acids to enhance anti-mold effects. Feeding formic acid-treated green feed to dairy cows can prevent winter milk production declines and significantly improve fattening effects. In the food industry, formic acid is used for disinfection and preservation in the brewing industry, as a cleaning and disinfection agent for canned foods, and as a preservative for juices and foods.
A large number of formic acid derivatives serve as intermediates in the production of pharmaceuticals, pesticides, dyes, fragrances, and solvents, used in the manufacture of camphor, aminopyrine, caffeine, vitamin B1, analgin, insecticides, powdered rust inhibitors, triazoles, dimethylformamide, and others. Among formic acid derivatives, substituted formamides and formate esters are most widely used in industry. (For derivatives of formamide, see the entry for that compound).
Formic acid esters have a wide range of applications in the fragrance industry, such as: ethyl formate—peach, berry, and other fruit essences; isopentyl formate—fruit essences and leather fragrances; hexyl formate—apple essence; heptyl formate—for fruit essences such as apricot, plum, and peach; decyl formate—for orange blossom essence and iris oil; benzyl formate—for floral essences such as jasmine and soap essences; cinnamyl formate—for essences such as jasmine and moonflower;
Citronellyl formate — used in rose, osmanthus, wild lily, and other fruit essences; Eugenyl formate — used in carnation essence, etc.; Geranyl formate — used in rose, orange blossom, geranium, and other fruit essences; Linalyl formate — used in lavender, bergamot, and other fruit essences; Methyl formate — cosmetic fragrances and spray fragrances; Phenethyl formate — fragrances for white rose, orchid, chrysanthemum, etc.; Thymol formate — cosmetic fragrances.
Packaging Method of Formic acid 64-18-6






Factory Show