Glycerol 56-81-5
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
Glycerol 56-81-5

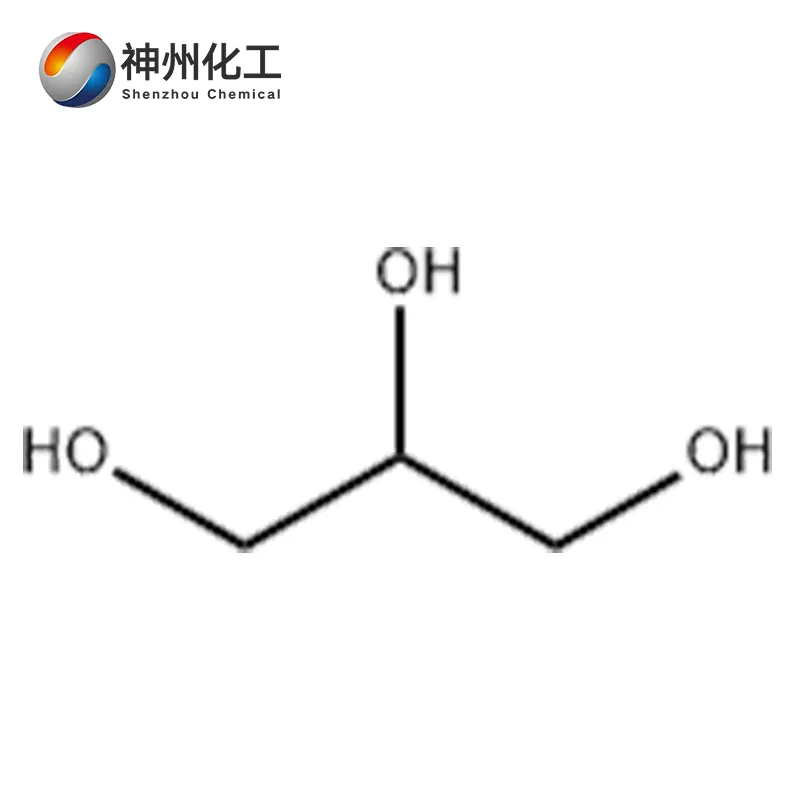


- Chemical Name:Glycerol
- CAS No.:56-81-5
- Product Categories:Food Additives
- Molecular Formula:C3H8O3
- Formula Weight:92.09
- Appearance:Colorless, viscous liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:Glycerol
- CAS No.:56-81-5
- Product Categories:Food Additives
- Molecular Formula:C3H8O3
- Formula Weight:92.09
- Appearance:Colorless, viscous liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available




Product Description of Glycerol 56-81-5
Glycerol 56-81-5 is a colorless, odorless, sweet-tasting viscous liquid. It has a boiling point of 290°C, a melting point of 17.9°C, and a relative density of 1.2613. It is completely miscible with water, and anhydrous glycerol has strong hygroscopic properties.
Glycerol has a weak acidic nature and can react with alkaline hydroxides. For example, when reacted with copper hydroxide, it forms bright blue glycerol copper (which can be used to identify polyols). Glycerol reacts with nitric acid to form trinitroglycerol, also known as nitroglycerin, a highly explosive substance. Due to its hygroscopic properties, glycerol is commonly used as a humectant and emollient in cosmetics, leather, tobacco, food, and textiles.
Glycerol also has a laxative effect and can be used for enemas or made into suppositories to treat constipation. Nitroglycerin has the effect of dilating coronary arteries and can be used to treat angina pectoris. Nitroglycerin can be used as an explosive and propellant. Glycerol reacts with dicarboxylic acids to produce aldehyde resins, which are widely used in paints and coatings. In nature, glycerin is widely present in the form of esters.
For example, various animal and plant fats are glycerin carboxylic acid esters, and hydrolyzing fats yields fatty acids and glycerin. Currently, one of the main sources of glycerin is a byproduct of the soap industry (hydrolysis of fats under alkaline conditions), and another source is the production of propylene from petroleum cracking gas.
Chemical Properties of Glycerol 56-81-5
| Melting point | 20 °C(lit.) |
| Boiling point | 290°C |
| Density | 1.25 g/mL(lit.) |
| Vapor density | 3.1 (vs air) |
| Vapor pressure | <1 mm Hg (20°C) |
| Refractive index | n20/D 1.474(lit.) |
| FEMA | 2525 | GLYCEROL |
| Flash point | 320 °F |
| Storage conditions | Store at +5°C to +30°C. |
| Solubility | H2O:5 M, 20°C,Clear, colorless |
| Acidity coefficient (pKa) | 14.15(at 25°C) |
| Form | Viscous liquid |
| Color | APHA: s10 |
| Specific gravity | 1.265 (15/15℃)1.262 |
| Odor | Tasteless |
| pH value range for acid-base indicator color change | 5.5-8 |
| pH value | 5.5-8 (25°℃, 5M in H2O) |
| Explosion limit value | 2.6-11.3%(V) |
| Fragrance type | odorless |
| Biological origin | synthetic (organic) |
| Water solubility | >500 g/L (20 °C) |
| Sensitivity | Hygroscopic |
| Maximum wavelength | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.04 |
| Merck | 144,484 |
| JECFA Number | 909 |
| BRN | 635685 |
| Dielectric constant | 47.0 (Ambient) |
| Exposure limit | OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| Stability | Stable. Incompatible with perchloric acid, lead oxide, acetic anhydride, nitrobenzene, chlorine, peroxides, strong acids, and strong bases. Flammable. |
| InChlKey | PEDCQBHIVMGVHV-UHFFFAOYSA-N |
| LogP | -2.32 |
| Surface tension | 64mN/m at 20°C |
| CAS Database | 56-81-5(CAS DataBase Reference) |
| NIST Chemical Substance Information | 1,2,3-Propanetriol(56-81-5) |
| EPA Chemical Substance Information | Glycerine (56-81-5) |
| Absorption | s0.02 at 280nm in H20 at 0.5M s0.07 at 260nm in H20 at 0.5M |
Application of Glycerol 56-81-5
The Functions and Benefits of Glycerin
Glycerin lubricates and stimulates the intestinal walls, softens stools, and facilitates their passage. It also has a dehydrating effect. When combined with sodium ascorbate into a compound formulation for intravenous administration, it can reduce intraocular pressure. Topically, it has a humectant effect and softens local tissues. It can dissolve boric acid, boric acid salts, phenol, nucleic acids, and salicylic acid. It is primarily used for constipation in children and the elderly, emergency treatment of general cerebral edema, glaucoma therapy, and winter skin cracking and peeling.
Humectant (used in bread, cakes, etc.); carrier solvent (used in spices, pigments, non-water-soluble preservatives, etc.); thickening agent (used in beverages, blended wines, etc.); plasticizers (for candies, desserts, meat products); sweeteners.
EEC regulations permit its use in alcoholic beverages, candies, cakes, coating finishes, meat and cheese coatings, non-alcoholic beverages, baked goods, chewing gum, gelatin sweets, etc.
Glycerol is an important basic organic raw material with widespread applications in industry, medicine, and daily life. Currently, it has approximately 1,700 uses, primarily in medicine, cosmetics, alkyd resins, tobacco, food, alkyd resins, celluloid, explosives, and textile dyeing. The consumption of glycerol in fields such as alkyd resins, celluloid, and explosives is declining. However, its application in pharmaceuticals, cosmetics, and food is expected to continue growing.
After hydrolysis in the body, it can serve as a nutritional source. It is also widely used in industries such as textiles, food, papermaking, metal processing, paint, daily chemicals, petrochemicals, pharmaceuticals, tobacco, and military.
Packaging Method of Glycerol 56-81-5






Factory Show