Tetrachloroethylene 127-18-4
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
Tetrachloroethylene 127-18-4

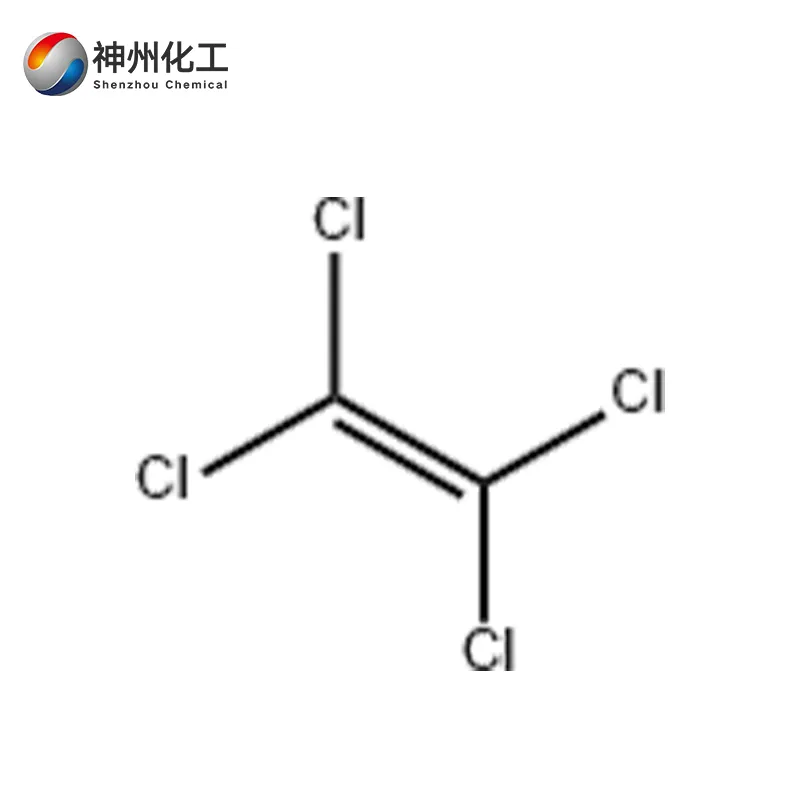


- Chemical Name:Tetrachloroethylene 127-18-4
- CAS No.:127-18-4
- Product Categories:Organic Chemistry
- Molecular Formula:C2Cl4
- Formula Weight:165.83
- Appearance:Colorless transparent liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:Tetrachloroethylene 127-18-4
- CAS No.:127-18-4
- Product Categories:Organic Chemistry
- Molecular Formula:C2Cl4
- Formula Weight:165.83
- Appearance:Colorless transparent liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available




Tetrachloroethylene 127-18-4
Tetrachloroethylene 127-18-4, also known as perchloroethylene, is a compound formed by replacing all hydrogen atoms in ethylene with chlorine atoms. It was first synthesized by Faraday in 1821 during the thermal decomposition of hexachloroethane.
It is a colorless, transparent liquid with an ether-like odor. It is non-flammable. Its relative molecular mass is 165.85. Relative density: 1.6220. Melting point: -22.7°C. Boiling point: 121.2°C, 33.2°C (4.000×10³ Pa). Refractive index: 1.5055. Viscosity: 0.839 mPa·s (20°C). Vapor pressure (×10³ Pa): 5.466 (40°C), 13.865 (60°C), 30.131 (80°C), 58.128 (100°C).
Tetrachloroethylene is insoluble in sugar, glycerol, and proteins, slightly soluble in water (0.015 at 25°C), and miscible with ethanol, ether, chloroform, benzene, and chlorinated organic solvents. It does not hydrolyze.
According to Chemicalbook, it remains stable even when heated to 500°C in the absence of air, moisture, and catalysts. Hydrogenation produces tetrachloroethane. Chlorination produces hexachloroethane.
Tetrachloroethylene can also react with bromine to form monobromotrichloromethane or dibromodichloromethane compounds. Under the action of a catalyst, it can also react with hydrogen fluoride.
When exposed to light, air, and water over an extended period, it slowly decomposes into trichloroacetaldehyde and phosgene, corroding metals such as iron, aluminum, and zinc.
This decomposition can be inhibited by adding stabilizers. In the presence of activated carbon, heating to 700°C causes it to decompose into hexachlorobenzene and hexachloroethane. Tetrachloroethylene can be oxidized by strong oxidizing agents. Tetrachloroethylene reacts violently with barium powder, beryllium powder, lithium shavings, nitrogen tetroxide, and sodium hydroxide.
Tetrachloroethylene is toxic and acts as a central nervous system depressant, causing headaches, nausea, vomiting, and even coma. The oral LD50 in mice is 8850 mg/kg.
The maximum allowable concentration in the workplace is 100 × 10⁻⁶.
Chemical Properties of Tetrachloroethylene 127-18-4
| Melting point | -22 °C (lit.) |
| Boiling point | 121 °C (lit.) |
| density | 1.623 g/mL at 25 °C (lit.) |
| vapor pressure | 13 mm Hg ( 20 °C) |
| refractive index | n20/D 1.505(lit.) |
| Fp | 120-121°C |
| storage temp. | Store at +2°C to +25°C. |
| solubility | Solubility in water: 0.15g/L, 25 ° C |
| form | clear transparent liquid |
| pka | / |
| color | colorless |
| Odor | Chloroform like odor |
| Water Solubility | Miscible with alcohol, ether, chloroform, benzene and hexane. Slightly miscible with water. |
| Merck | 14,9190 |
| BRN | 1361721 |
| Dielectric constant | 2.5(21℃) |
| InChIKey | / |
| LogP | 2.53 at 20℃ |
| CAS DataBase Reference | 127-18-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetrachloroethylene(127-18-4) |
| EPA Substance Registry System | Tetrachloroethylene (127-18-4) |
| Hazard Codes | Xn,N,T,F |
| Risk Statements | 40-51/53-23/25-11-39/23/24/25-23/24/25 |
| Safety Statements | 23-36/37-61-45-24-16-7 |
| RIDADR | UN 1897 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | KX3850000 |
| Autoignition Temperature | 260℃ |
| TSCA | Yes |
| HS Code | 29032300 |
| Hazardous Substances Data | 127-18-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in mice: 8.85 g/kg (Dybing); LC for mice in air: 5925 ppm (Lazarew) |
Application of Tetrachloroethylene 127-18-4
Tetrachloroethylene is used as an organic solvent, dry cleaning agent, oil extractant, smoke screen agent, desulfurization agent, and fabric finishing agent.
It has a wide range of applications, primarily as an organic solvent, dry cleaning agent, and metal degreasing solvent. It is also used as a solvent for anthelmintic drugs and as a standard substance for chromatographic analysis.
Tetrachloroethylene can be used as a fat extractant, fire extinguishing agent, and smoke screen agent, among other applications. It is also used in the synthesis of trichloroethylene and fluorinated organic compounds.
In organic analysis, it serves as a solvent for fats or fat-like substances. It is also used as a high-pressure liquid chromatography reagent and a solvent for spectrophotometric determinations. Additionally, it is employed in organic synthesis.
Packaging Method of Tetrachloroethylene 127-18-4






Factory Show