2-Ethylhexanol 104-76-7
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
2-Ethylhexanol 104-76-7
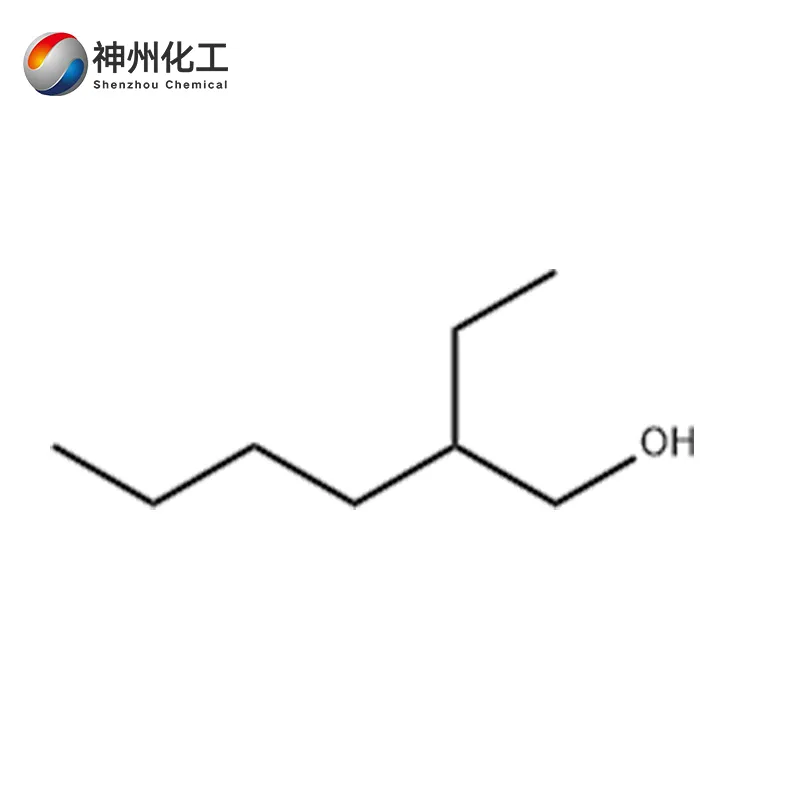


- Chemical Name:2-Ethylhexanol
- CAS No.:104-76-7
- Product Categories:Organic Chemistry
- Molecular Formula:C8H18O
- Formula Weight:130.23
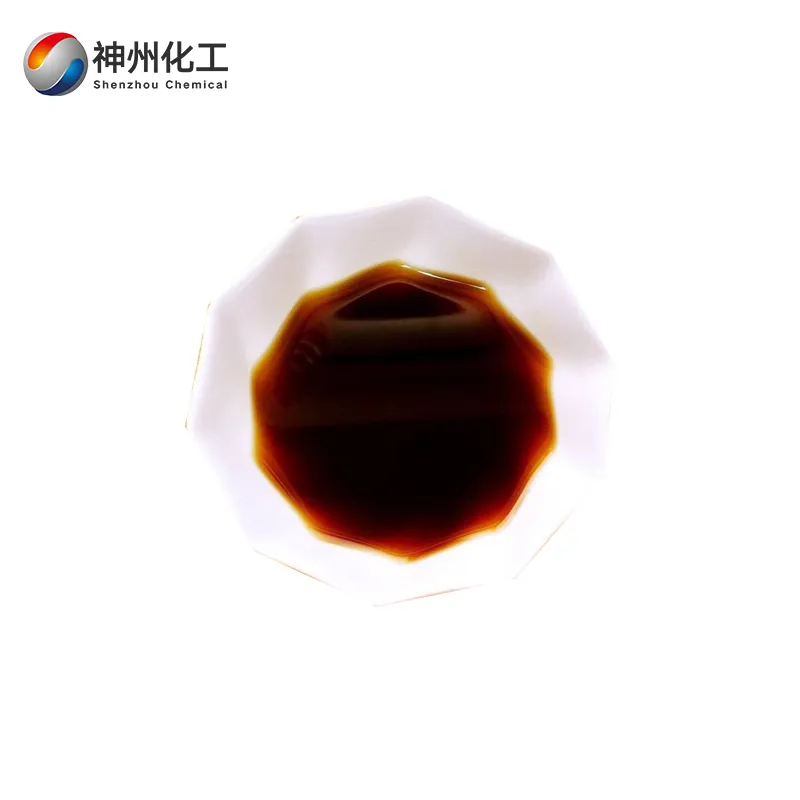
- Appearance:Dark brown liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:2-Ethylhexanol
- CAS No.:104-76-7
- Product Categories:Organic Chemistry
- Molecular Formula:C8H18O
- Formula Weight:130.23
- Appearance:Dark brown liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available




2-Ethylhexanol 104-76-7
1. Synthesis of 2-Ethyl-2-hexenal Add 30 g (0.42 mol) of butyraldehyde to a 250 mL three-neck flask, a 5% sodium hydroxide solution (15 mL), and ethanol (50 mL). Stir the mixture in a water bath at 40°C overnight. Monitor the reaction by thin-layer chromatography (TLC).
Remove the ethanol by rotary evaporation. Extract the mixture three times with 100 mL of ethyl acetate, combine the organic layers, wash with distilled water, rinse with brine, dry with anhydrous magnesium sulfate, filter and evaporate to dryness, yielding a pale yellow transparent liquid (13.8 g, 52% yield).
2. Synthesis of 2-ethyl-2-hexen-1-ol Add 2-ethyl-2-hexenal (5 g, 40 mmol), sodium borohydride (3 g, 80 mmol), and anhydrous methanol (20 mL) to a 100 mL three-neck flask.
Stir at room temperature for 3 hours. Add 10 mL of pure water under ice bath conditions, extract with ethyl acetate, combine the organic layers, wash with pure water, wash with saturated brine, dry with anhydrous magnesium sulfate, filter, spin-dry, and purify by column chromatography to obtain a colorless transparent liquid (4.36 g), yield: 85%.
3. Synthesis of (S)-2-ethylhexanol Under a nitrogen atmosphere, add the biphasic ligand ChenPhos (3.28 mg, 0.0021 mmol), Rh(NBD)₂BF₄ (1.50 mg, 0.002 mmol), and dichloromethane (2 mL) into a 10 mL reaction vessel under a nitrogen atmosphere, stirred for 30 minutes, yielding a yellow, clear solution, which serves as the catalyst.
Add 2-ethyl-2-hexenol (25.6 mg, 0.2 mmol), then place the inner tube in a hydrogenation reactor. Perform three hydrogenation and degassing operations to replace the atmosphere with hydrogen, then pressurize to 50 atm and stir at room temperature for 20 hours. Stop the reaction, release the gas, and concentrate the reaction mixture by rotary evaporation. Filter through a silica gel column to obtain a colorless oily liquid (25.2 mg), with a yield of 97% and 98.3% ee.
Chemical Properties of 2-Ethylhexanol 104-76-7
| Melting point | -76 °C (lit.) |
| Boiling point | 183–186 °C (lit.) |
| Density | 0.833 g/mL at 25 °C (lit.) |
| Vapor density | 4.49 (vs air) |
| Vapor pressure | 0.2 mm Hg (20°C) |
| Refractive index | n20/D 1.431 (lit.) |
| FEMA | 3151 | 2-Ethyl-1-hexanol |
| Flash point | 171 °F |
| Storage conditions | Store below +30°C. |
| Solubility | Soluble in water |
| Form | Liquid |
| Acidity coefficient (pKa) | 15.05±0.10 (Predicted) |
| Color | Transparent |
| pH value | 7 (1 g/L, H₂O, 20°C) |
| Odor | Sweet. |
| Biological source | Synthetic |
| Olfactory threshold | 0.013 ppm |
| Fragrance type | Citrus |
| Explosion limit values | 0.88%, 104°F |
| Water solubility | 1 g/L (20°C) |
| JECFA Number | 267 |
| Merck | 143,808 |
| BRN | 1719280 |
| Dielectric constant | 3.4 (18°C) |
| Stability | Stable. Flammable. Incompatible with strong oxidizers and strong acids. |
| InChlKey | YIWUKEYIRIRTPP-UHFFFAOYSA-N |
| LogP | 2.9 at 25°C |
| CAS Database | 104-76-7(CAS DataBase Reference) |
| NIST Chemical Substance Information | 1-Hexanol, 2-ethyl-(104-76-7) |
| EPA Chemical Substance Information | 2-Ethylhexanol (104-76-7) |
Application of 2-Ethylhexanol 104-76-7
GB2760–1996 Permitted Food Flavoring Agents.
2-Ethylhexanol can be used in the production of plasticizers, defoamers, dispersants, mineral processing agents, and petroleum additives, as well as in dyeing, painting, and film applications.
2-Ethylhexanol is commonly referred to as octanol in the plasticizer industry and is an important chemical raw material. Overseas, octanol is not only used for producing plasticizers but also for manufacturing octyl acrylate or as a surfactant, among other applications.
It is primarily used to produce: Dioctyl phthalate (DOP), dioctyl adipate (DOA), trioctyl trimellitate (TOTM), other plasticizers, octyl acrylate, surfactants, lubricant additives, mining applications, diesel fuel additives, solvents and formulations, rust inhibitors, and other chemicals.
Octanol itself is a useful solvent, defoamer, dispersant, and lubricant, with its main products being phthalic acid di-octyl ester and other plasticizers, as well as octyl acrylate. Phthalic acid di-octyl ester produced from octanol is the primary plasticizer for PVC, accounting for approximately 95% of its total consumption.
Packaging Method of 2-Ethylhexanol 104-76-7






Factory Show