Calcium Chloride Anhydrous 10043-52-4
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
Calcium Chloride Anhydrous 10043-52-4

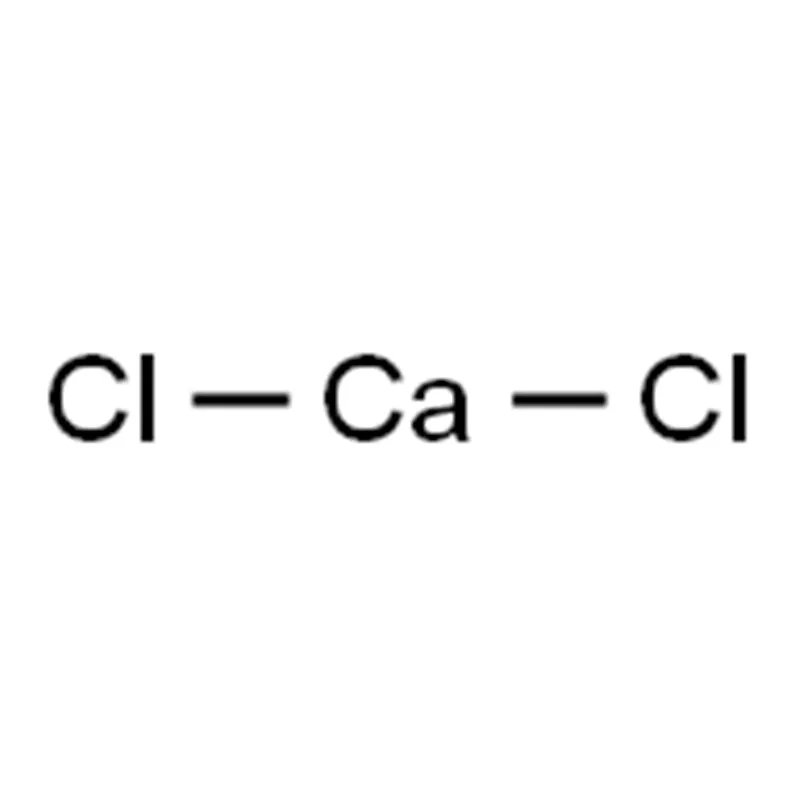


- Chemical Name:Calcium Chloride Anhydrous
- CAS No.:10043-52-4
- Product Categories:Inorganic Chemistry
- Molecular Formula:CaCl2
- Formula Weight:110.98
- Appearance:powder
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:Calcium Chloride Anhydrous
- CAS No.:10043-52-4
- Product Categories:Inorganic Chemistry
- Molecular Formula:CaCl2
- Formula Weight:110.98
- Appearance:powder
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available

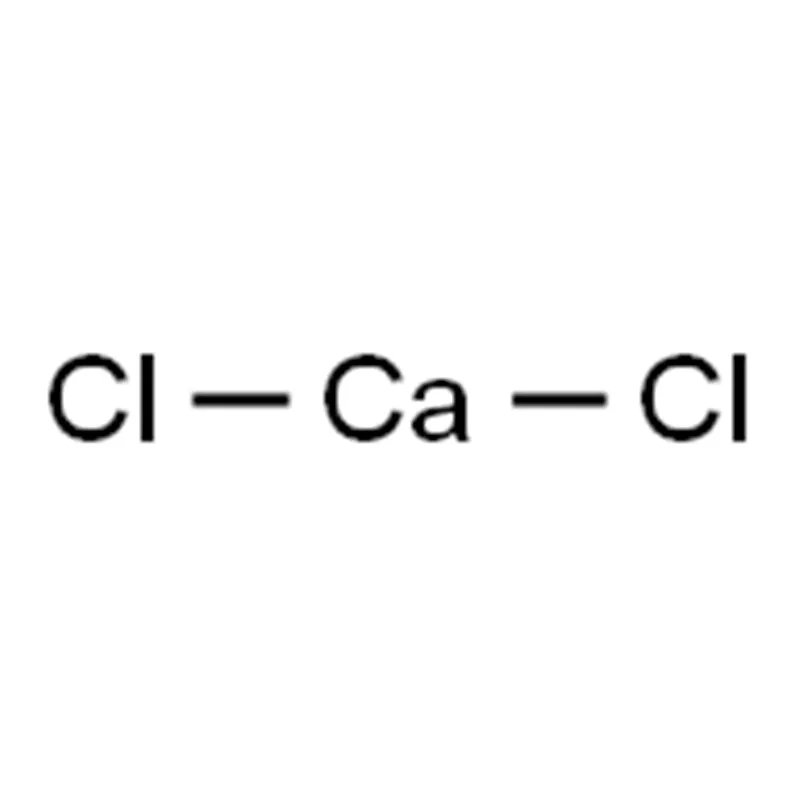


Product Description Of Calcium Chloride Anhydrous 10043-52-4
Calcium Chloride Anhydrous 10043-52-4 is a white, porous, crystalline solid or granule. It is highly hygroscopic. Its melting point is 782°C, density is 2.15 g/cm³, boiling point exceeds 1600°C, it is highly soluble in water with the release of a large amount of heat, and also soluble in ethanol and acetone. The most common form is hexahydrate calcium chloride (CaCl₂·6H₂O), which is a colorless trigonal crystal, hygroscopic, with a bitter-salty taste, and a density of 1.71 g/cm³. It dissolves in crystalline water at 29.92°C. When heated to 30°C, it loses four molecules of water to form the dihydrate (CaCl₂·2H₂O), a white, porous, hygroscopic solid. Further heating can produce the monohydrate. At temperatures above 200°C, it completely loses water to form anhydrous calcium chloride, which is highly hygroscopic. Calcium chloride reacts with ammonia to form the ammonium chloride hydrate CaCl₂·8NH₃.
Chemical Properties Of Calcium Chloride Anhydrous 10043-52-4
| Melting point | 772 °C (lit.) |
| Boiling point | 1935 °C/1 atm (lit.) |
| Density | 1.086 g/mL at 20 °℃ |
| Vapor pressure | 0.01 mm Hg (20°C) |
| Refractive index | n20/D 1.358 |
| Flash point | >1600°℃ |
| Storage conditions | Store at +5°C to +30°C. |
| Solubility | Soluble in water |
| Form | Powder |
| Color | White to gray |
| Specific gravity | 2.15 |
| Odor | Odorless |
| Flame color | Redish |
| pH value | 8-10 (100g/l, H2O, 20℃) |
| Water solubility | 740 g/L (20 °C) |
| Sensitivity | Hygroscopic |
| Maximum wavelength (λmax) | λ: 260 nm Amax: 0.04 |
| Crystal structure | λ: 280 nm Amax: 0.02 |
| Crystal system | CaCI2 type |
| Merck | Nogata |
| Space group | 141,659 |
| Stability | Pnnm |
| InChlKey | Stable. Incompatible with zinc, water, strong acids, methyl vinyl ether, bromine trifluoride, boron oxide, and calcium oxide. Hygroscopic. |
| CAS Database | 10043-52-4(CAS DataBase Reference) |
| NIST Chemical Substance Information | Calcium dichloride(10043-52-4) |
| EPA Chemical Substance Information | Calcium chloride (10043-52-4) |
Application of Calcium Chloride Anhydrous 10043-52-4
Calcium Chloride Anhydrous 10043-52-4 is used in brine for refrigeration equipment, road de-icing agents, and desiccants. Since it readily absorbs moisture and hydrates in the air, anhydrous calcium chloride should be stored in sealed containers. Calcium chloride, its hydrates, and solutions have significant applications in food manufacturing, building materials, medicine, and biology.
Anhydrous Calcium Chloride Anhydrous is a commonly used desiccant and drying agent in industrial production and laboratories (but it cannot be used to dry ammonia, hydrogen sulfide, or alcohol). It is primarily used for drying gases, petroleum, and organic solvents. In the inorganic industry, it serves as raw material for producing metallic calcium and various calcium salts. Calcium chloride is also used as a sizing agent for textiles, a water purifier, an antifreeze agent, a food preservative, and a road cleaning agent. When mixed with ice in a 1.44:1 ratio, CaCl₂·6H₂O is used as a refrigerant in laboratories and is also an important refrigerant in the refrigeration industry, capable of achieving a low temperature of -54.9°C.
Application: Calcium Chloride Anhydrous is composed of chlorine and calcium, with the chemical formula CaCl2. It is a typical ionic halide, appearing as a white solid at room temperature, with its aqueous solution being neutral. Calcium chloride, its hydrates, and solutions have significant application value in various fields such as food manufacturing, building materials, medicine, and biology.
Packaging Method Of Calcium Chloride Anhydrous 10043-52-4






Factory Show