Ethylene glycol 107-21-1
Have Any Questions?
Let our vertically integrated solutions – from Chinese manufacturing hubs to your local warehouse – become your competitive advantage.
- +86 13376344351
Leave Your Message
Ethylene glycol 107-21-1



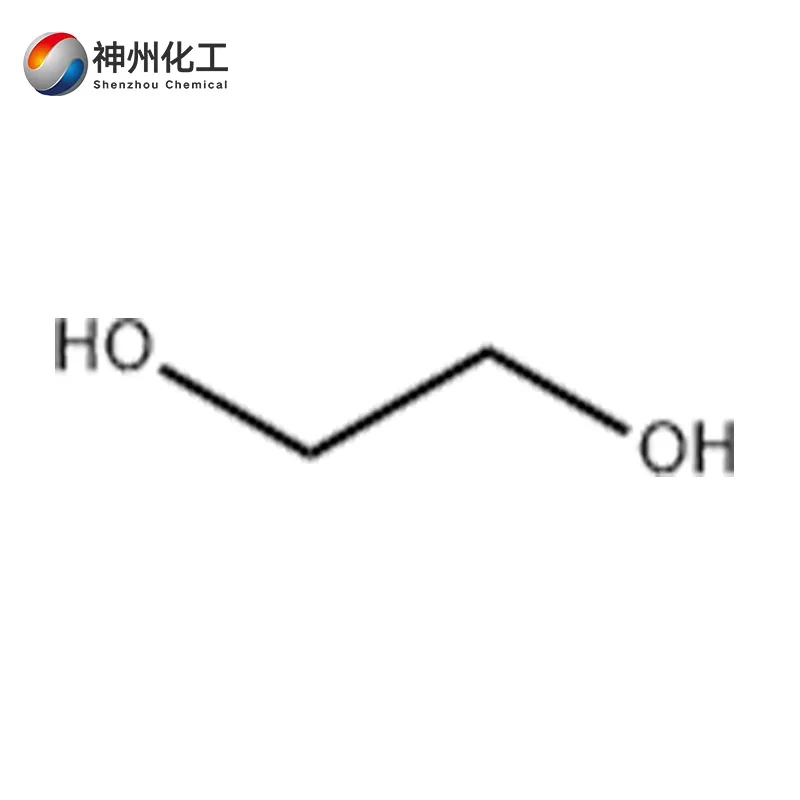
- Chemical Name:Ethylene glycol
- CAS No.:107-21-1
- Product Categories:Organic Chemistry
- Molecular Formula:C2H6O2
- Formula Weight:62.07
- Appearance:Colorless transparent viscous liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available
- Chemical Name:Ethylene glycol
- CAS No.:107-21-1
- Product Categories:Organic Chemistry
- Molecular Formula:C2H6O2
- Formula Weight:62.07
- Appearance:Colorless transparent viscous liquid
- Storage and transportation characteristics: low temperature, ventilation, dryness, waterproof, moisture-proof
- Type Of Transportation:By Air/By Sea/By Train/By Express
- Type Of Transportation:Available




Product Description Of Ethylene glycol 107-21-1
Ethylene glycol, also known as glycol, is the simplest aliphatic diol and exhibits the chemical properties of alcohols, such as the ability to form ethers and esters, to be oxidized into aldehydes or acids, and to condense into ethers. It can also be substituted by halogens. When reacted with acyl chlorides or acid anhydrides, it generally forms diesters.
Under the action of a catalyst (manganese dioxide, aluminum oxide, zinc oxide, or sulfuric acid), heating can cause dehydration within or between molecules, forming cyclic ethylene glycol acetaldehyde. This reacts with nitric acid to produce ethylene glycol dinitrate (an explosive).
Ethylene glycol is a raw material for the production of polyester resins, alkyd resins, and polyester fibers, and is also used as an automotive antifreeze and aircraft engine coolant. In 1980, the consumption of ethylene glycol used as antifreeze in the United States was equal to that used for polyester production.
Additionally, it can be used to synthesize high-molecular-weight compounds such as polyester fibers. Ethylene glycol dinitrate, when used in combination with nitroglycerin, can lower the freezing point of explosives. Ethylene glycol can also serve as a raw material for pharmaceuticals and plastics, as well as a high-boiling-point solvent. Industrially, it is produced from ethylene, which is first converted into ethylene oxide and then hydrolyzed to yield ethylene glycol. This product poses risks of fire and explosion.
It is irritating to the skin and mucous membranes. Inhalation of vapors or dermal absorption can cause anesthetic effects on the central nervous system and renal dysfunction. The oral LD50 in rats is 8540 mg/kg. The maximum allowable concentration in the workplace is 5 × 10⁻⁶.
Chemical Properties Of Ethylene glycol 107-21-1
| Melting point | -13 °C (lit.) |
| Boiling point | 195-198 °C |
| density | 1.113 g/mL at 25 °C (lit |
| vapor pressure | 0.08 mm Hg ( 20 °C) |
| refractive index | n20/D 1.431(lit.) |
| Fp | 230 °F |
| storage temp. | 2-8°C |
| solubility | Water: miscible |
| form | viscous liquid |
| pka | 14.22(at 25℃) |
| color | colorless |
| Odor | tasteless |
| Water Solubility | miscible |
| Merck | 14,3798 |
| BRN | 505945 |
| Dielectric constant | 37.0(20℃) |
| InChIKey | / |
| LogP | -1.36 at 25℃ |
| CAS DataBase Reference | 107-21-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Ethanediol(107-21-1) |
| EPA Substance Registry System | Ethylene glycol (107-21-1) |
Application of Ethylene glycol 107-21-1
Ethylene glycol is primarily used in the formulation of antifreeze for automotive cooling systems and in the production of polyethylene terephthalate (a raw material for polyester fibers and plastics). It is also used in the production of synthetic resins, solvents, lubricants, surfactants, softeners, humectants, explosives, and other products.
Ethylene glycol can often be used as a substitute for glycerin, serving as a hydrating agent in the leather industry and a solvent in the pharmaceutical industry. Ethylene glycol has strong solubility, but it is easily metabolized and oxidized to form toxic oxalic acid, so it cannot be widely used as a solvent. Adding ethylene glycol to hydraulic fluids can prevent oil-based hydraulic fluids from corroding rubber components in the system; water-based hydraulic fluids with ethylene glycol as the main component are non-flammable hydraulic fluids used in aircraft, automobiles, and high-temperature molding machines.
Ethylene glycol has many important derivatives. Low-molecular-weight polyethylene glycols (ethylene glycol monomethyl ether, ethylene glycol dimethyl ether, ethylene glycol trimethyl ether, or alternatively referred to as diethylene glycol, triethylene glycol, and tetraethylene glycol) are actually byproducts of ethylene glycol produced by the hydration of ethylene oxide. Diethylene glycol can be used as a humectant, plasticizer, sizing agent, printing ink solvent, natural gas dehydration agent, and aromatic extraction solvent.
Diethylene glycol dinitrate, similar to ethylene glycol dinitrate, is also an important industrial explosive. High-molecular-weight polyethylene glycols, depending on their molecular weight, range from colorless, transparent, viscous liquids to waxy solids and are also a useful class of derivatives. They are used as lubricants, moisture retainers, solvents, and intermediates in the rubber and food industries, as well as in the formulation of cosmetics and as additives in the textile and paper industries.
Ethylene glycol esters are widely used as solvents. Ethylene glycol esters of long-chain fatty acids have surface-improving properties and can be used alone or in combination with surfactants as emulsifiers, stabilizers, dispersants, humectants, foaming agents, and suspending agents. Ethylene glycol reacts with urea to form cyclohexylene urea, which is used in the textile industry. Ethylene glycol disodium reacts with 1,2-dibromoethane to form dioxane, a special solvent.
Different oxidizing agents or reaction conditions are used for ethylene glycol. After oxidation, products such as ethylene glycol aldehyde, ethylene glycol, ethylene glycol acid, and oxalic acid can be obtained.
Packaging Method of Ethylene glycol 107-21-1






Factory Show